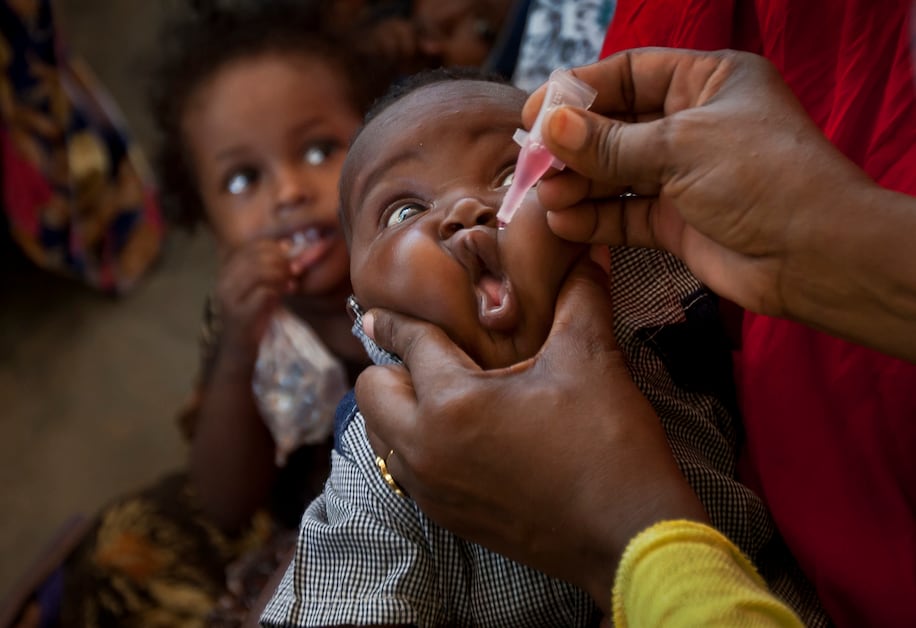
The World Health Organization (WHO) has officially prequalified the novel type 2 oral polio vaccine (nOPV2) for global use.
The vaccine protects children against polio. The pre-qualification of the vaccines is very important, as it was granted Emergency Use Listing (EUL) three years ago.
The vaccine, which was developed with contributions from scientists at the UK Medicines and Healthcare Products Regulatory Agency (MHRA), after a series of tests, proved to be safe and effective.
This is coming after the WHO facilitated the worldwide distribution of 950 million doses of the vaccines.
Lafiya360 reports that nOPV2 is a modified version of the oral polio vaccine (OPV), which targets poliovirus type 2.
This novel vaccine would reduce the risk of vaccine-derived outbreaks, which has been a source of concern for some time now. The concern was that the weakened virus in the vaccine circulates in under-immunized populations, potentially regaining the ability to cause paralysis.
The nOPV2, as a shield against polio for children, has now received pre-qualification status from the WHO, streamlining access for member countries without the stringent criteria previously mandated under the EUL.
This pre-qualification ensures broader availability for global organizations to supply and distribute the nOPV2 in developing nations.